Background
In the 1970s, the aminoindanes were reported to possess significant bronchodilating and analgesic properties [1]. Potent effects on the release and re-uptake of serotonin were also observed [2]. As a result of the latter these substances have been sold as NPS for their ability to produce empathogenic and entactogenic effects of serotonin releasing drugs, such as MDMA [3].
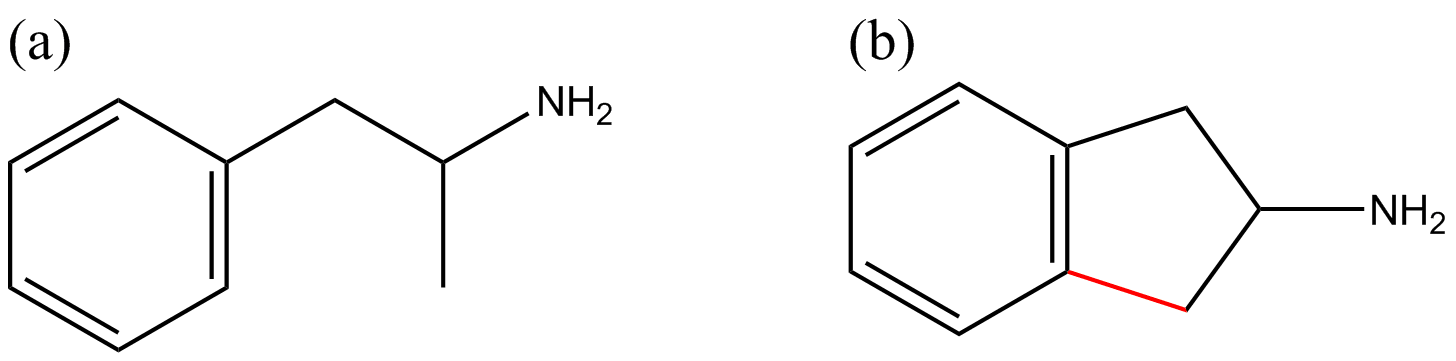
Figure 1 Chemical structures of amphetamine (a) and 2-aminoindane (2-AI) (b). The structural difference between amphetamine (internationally controlled substance) and 2-AI is highlighted in red.
The prototype aminoindane, 2-aminoindane (2-AI), is a cyclic analogue of amphetamine (see Figure 1). The 2-AI backbone structure can be modified to produce diverse chemical substances by substitution on the aromatic ring with a variety of functional groups, or the addition of a methylenedioxy bridge or N-alkylation; generating the following substances, respectively: 5-iodo-2-aminoindane (5-IAI, Figure 2c), 5,6-methylenedioxy-2-aminoindane (MDAI, Figure 2b) and N-methyl-2-aminoindane (NM-2AI, Figure 2d). Analogues of aminoindanes can be prepared using indanone, indene or after intramolecular cyclization of the acyl chloride derivative of 3-phenyl-2-propanoic acid [4].
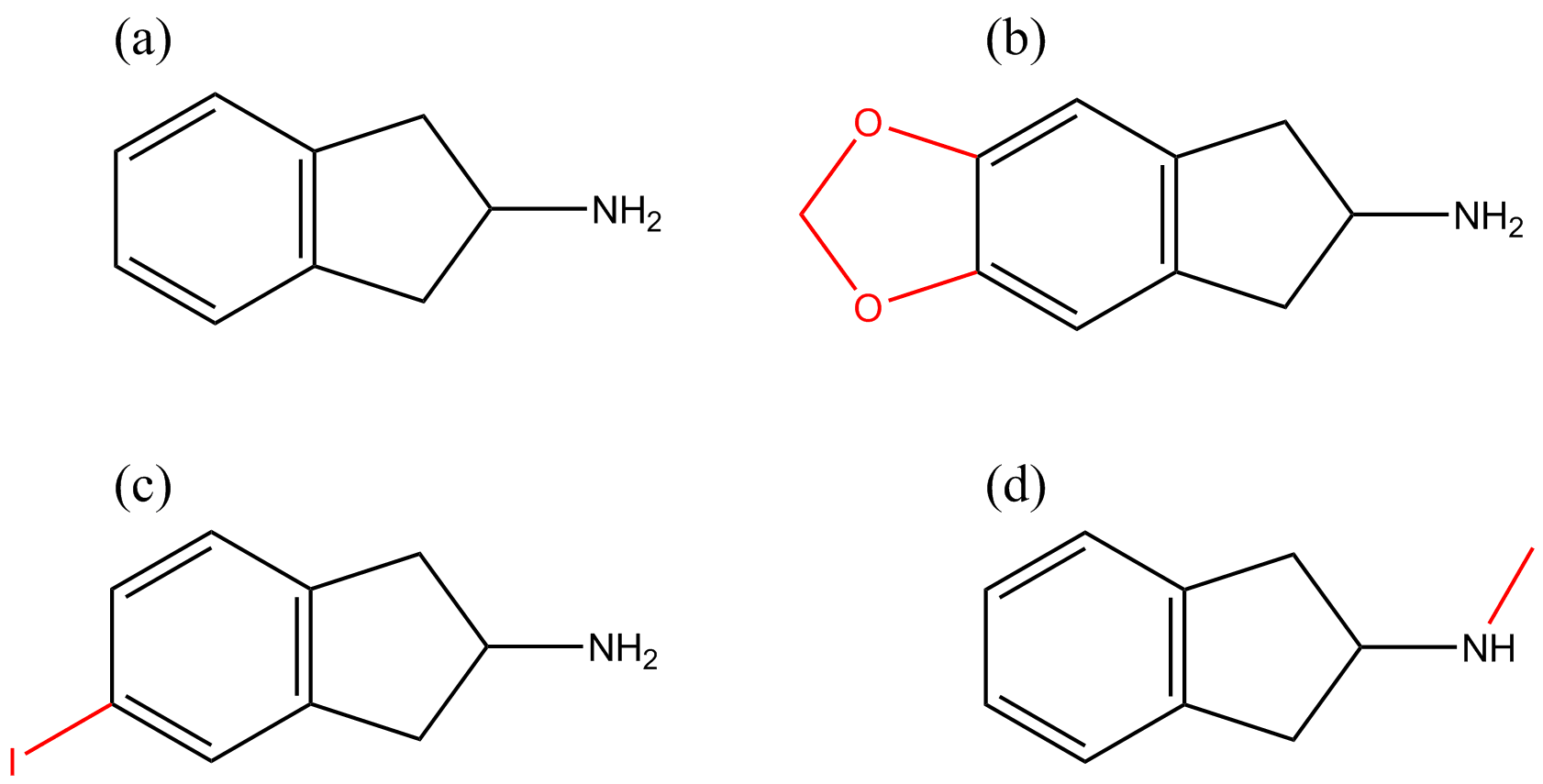
Figure 2 Chemical modifications of the 2-AI backbone (a) are indicated in red: addition of methylenedioxy bridge (e.g. MDMAI) (b), aromatic ring-substitution (e.g. 5-IAI) (c) and N-alkylation (e.g. NM-2AI) (d).
Other aminoindanes sold as NPS include highly potent selective serotonin releasing agents such as MDAI and 5-IAI, and ETAI (N-ethyl-5-trifluoromethyl-2-aminoindane) [4] which is an analogue of fenfluramine, a substance formerly marketed as an appetite suppressant [5, 6, 7].
Until May 2015, MDAI, 5-IAI and 2-AI were the most commonly reported substances in this group by Member States. In recent years there have been an increased number of reports to the UNODC EWA of substances with N-alkylation of the 2-AI backbone, such as NM-2AI (Figure 2d).
None of the aminoindanes are under international control.
Description
Street names of MDAI include ‘MDAI gold’, while 2-AI has been found in party pills known as ‘Pink Champagnes’ [8]. Aminoindanes are commonly found in powder form and crystals and are usually ingested, but snorting is also possible.
Aminoindanes act predominantly as central nervous system stimulants. Stimulants mediate the actions of dopamine, norepinephrine and/or serotonin, mimicking the effects of traditional drugs such as cocaine, amphetamine, methamphetamine, and ecstasy.
Reported adverse effects
Research conducted in animals and in in vitro cell cultures indicates that aminoindanes are relatively benign at recreational doses; however, the effects on humans have not yet been reported [4]. Animal studies have shown that these substances did not present any long-term neurotoxicity at the levels administered, [2,3,9] but slight neurotoxicity in rodents was shown after administration of very high doses of 5-IAI [10].
For further details regarding chemical structures, production and analysis see also the UNODC report The challenge of new psychoactive substances (click here).
References
[1] E. Solomons and J. Sam, “2-aminoindans of pharmacological interest”, Journal of Medicinal Chemistry 16 (1973): 1330-33.
[2] M.P. Johnson, S.P. Frescas, R. Oberlender and D.E. Nichols, “Synthesis and pharmacological examination of 1-(3-methoxy-4-methylphenyl)-2-aminopropane and 5-methoxy-6-methyl- 2-aminoindan: similarities to 3,4-(methylenedioxy)methamphetamine (MDMA)”, Journal of Medicinal Chemistry 34 (1991): 1662-8.
[3] A.P. Monte, D. Marona-Lewicka, N.V. Cozzi and D.E. Nichols, “Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogs of 3,4-(methylenedioxy)amphetamine”, Journal of Medicinal Chemistry 36 (1993): 3700-6.
[4] P.D. Sainsbury, A.T. Kicman, R.P. Archer, L.A. King and R.A. Braithwaite “Aminoindanes - the next wave of ‘legal highs’?”, Drug Testing and Analysis 3 (2011): 479-482.
[5-7] Fenfluramine (Pondimin™) and (+)-fenfluramine (Redux™) were approved for the treatment of obesity by the United States Food and Drug Administration in 1973 and 1996, respectively. Both fenfluramines were withdrawn from the market in 1997 because valvular heart disease (VHD) was discovered in some patients receiving these drugs;
[5] H.M. Connolly, J.L. Crary, M.D. McGoon, D.D. Hensrud, B.S. Edwards, W.D. Edwards and H.V. Schaff, “Valvular heart disease associated with fenfluramine-phentermine”, New England Journal of Medicine 337 (1997): 581-8.
[6] H.M. Connolly and M.D. McGoon, “Obesity drugs and the heart”, Current Problems in Cardiology 24 (1999): 745-92.
[7] N.J. Weissman, “Appetite suppressants and valvular heart disease”, The American Journal of the Medical Sciences 321 (2001): 285-91.
[8] P.V. Kavanagh, J. Sharma, S. McNamara, D. Angelov, S. McDermott, D. Mullan and S. Ryder, Head shop ‘legal highs’ active constituents identification chart (May 2010, pre-ban).
[9] D. Marona-Lewicka, G.S. Rhee, J.E. Spraque and D.E. Nichols, “Reinforcing effects of certain serotonin-releasing amphetamine derivatives”, Pharmacology Biochemistry and Behavior 53 (1996): 99-105.
[10] D.E. Nichols, M.P. Johnson and R. Oberlender, “5-iodo-2-aminoindan, a nonneurotoxic analogue of p-iodoamphetamine”, Pharmacology Biochemistry and Behavior 38 (1991): 135-139.